Is Human Evolution To Blame As To How We Respond To The Foods We Crave?
Have you wondered why you prefer sweet over salty foods? Thanks to nutritional genomics, the scientific relationship between nutrition and genes, we are closer to understanding what really drives our eating habits.
Our eating behaviours are determined by complex interactions between our genes, physiological, psychological and social factors. As more research unfolds, we are starting to understand genetics plays a larger role than previously thought in determining our food intake and food preferences.
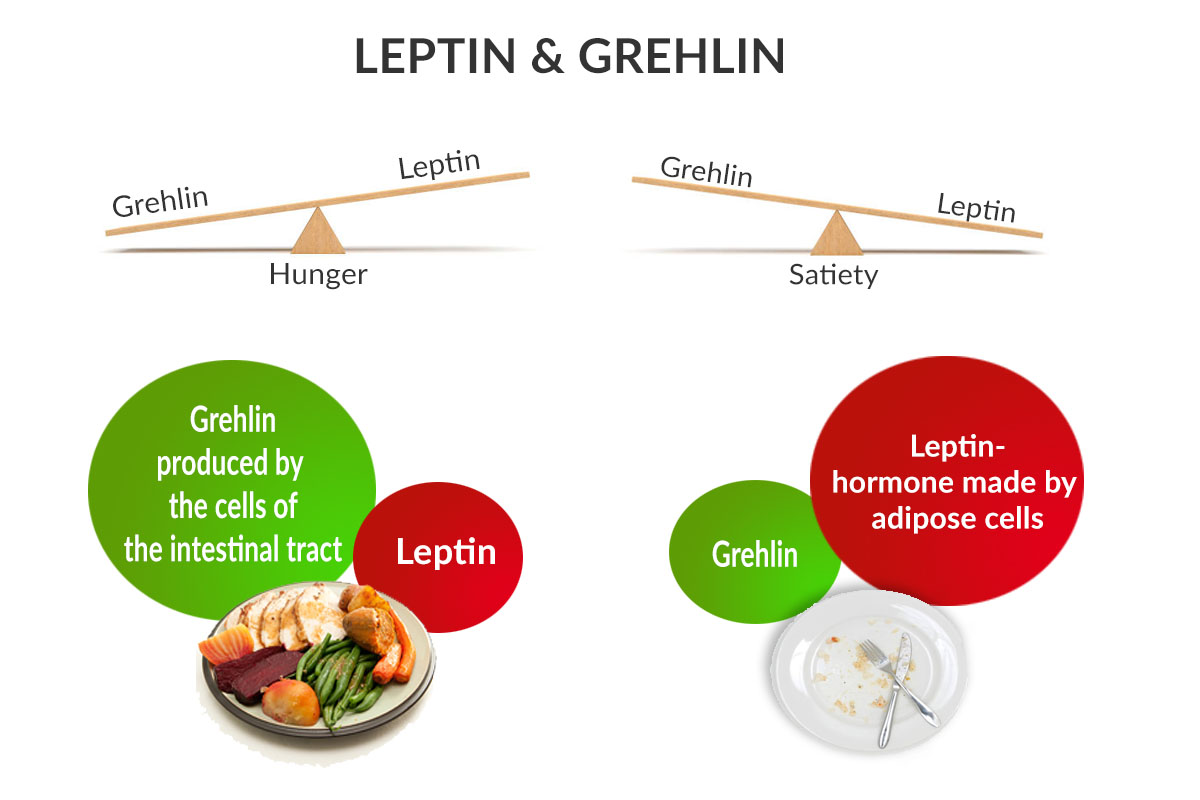
So, what happens when we are hungry? When our stomach is empty, the body releases a hormone called ghrelin (your hunger hormone) which interacts in the brain with a neurotransmitter called Neuropeptide Y (NPY) which encourages us to eat. Therefore NPY, which is the most abundant neuropeptide in the brain, has an important role in the stimulation of the appetite. Once we have satisfied our appetite by eating, this signals our ghrelin levels to drop and hunger subsides.
Conversely, after eating, a different hormone called leptin (our fullness or satiety hormone) is released from fat cells. Leptin interferes with NPY which then further suppresses the desire to eat.
So, with the hunger regulation system in place, why can’t we say no to that piece of cake or second helping of fries? Many food psychologists believe that in the past, energy rich foods such as those with lots of fat and sugar were hard to come by as hunter-gatherers so the human body needed to take advantage of these meals for survival. As a result, these fatty and sugary foods became extremely desirable.
Genetics of Eating Behaviour
The Fat and Obesity (FTO) and Melanocortin 4 receptor (MC4R) are both genes which have been associated with food cravings, including both our food choices and how much we are likely to eat. These genes are also known to be related to how we store fat.
Fat and Obesity (FTO) Gene
In the brain the FTO protein influences the signalling of a nutrient sensor, meaning your body may not know if you’ve eaten enough. Outside the brain, FTO protein is thought to affect the expression of ghrelin influencing the amount of it in your system1. Neither pathway is fully understood but research suggests how this protein might be related to cravings. FTO also affects other hormones such as leptin and adiponectin which is involved in breaking down fatty acids and controlling glucose levels.
A well-established genetic variant in FTO is known to have some control over FTO protein expression levels. Those with the variants AT or AA are associated with increased amounts of the FTO protein and are likely to eat more energy dense foods and have larger meal sizes increasing their risk of obesity. Lifestyle and dietary interventions such as physical exercise have been found to decrease this risk of obesity by up to 30 percent2 and cravings can be reduced through higher protein diets3. There is also a possible mechanism for how green tea extract might reduce the amount of the FTO protein reducing its effects4,5. So, exercise and green tea may be useful tips to pass on to your clients who have this genetic variant.
Melancortin 4 Receptor (MC4R) Gene
The MC4R gene expresses the Melanocortin 4 (MC4) protein which is linked to many functions including emotional responses, insulin sensitivity and lipid metabolism. It is most notably associated with obesity6,7. The Alpha -melanocyte-stimulating hormone (α-MSH) binds to MC4 protein which decreases your desire to eat8. Genetic variants of MC4R gene generally have less of this protein leading to higher food intakes and interestingly, research has shown preferences for higher fat foods9. Additionally, teenage homozygous MC4R carriers (two copies of the variant G) who skip meals are three times more likely to be obese10.
Low density lipoprotein (LDL) cholesterol also has a link to MC4 protein. In the hypothalamus, the area of the brain that regulates appetite, insulin deficient mice had decreased LDL cholesterol in their cell membrane leading to increased food intake and weight gain11. The MC4 protein can become non-functional if it remains at the cell membrane for too long. When LDL is depleted, MC4R can no longer be taken into the cell which eventually leads to less functional MC4R, so the cell becomes less sensitive to α-MSH causing less satiety from food.
MC4R is suspected of influencing emotional eating. In a study of 184 women, homozygous MC4R carriers felt less control over their eating. The same study even found that in brain scans of women there is lower grey matter volume in parts of homozygous carrier’s brains responsible for emotions, memory, and decision making12. Those with the GG variant should be more careful and disciplined when following diets.
What does this mean?
Fast forward to today’s world where these energy rich fatty and sugar foods are in abundance, we can see that our initial evolutionary desire is now plagued by our ability to choose wisely and eat healthy. This continual intake of fat and sugar overrides the regulatory system and is insufficient to control our typical Western diet meaning our genetic capacity to adapt is being surpassed and contributing to the obesity epidemic.
References
- Gulati, P., & Yeo, G. S. H. (2013). The biology of FTO: from nucleic acid demethylase to amino acid sensor. Diabetologia, 56(10), 2113–2121. http://doi.org/10.1007/s00125-013-2999-5
- Kilpeläinen, T. O., Qi, L., Brage, S., Sharp, S. J., Sonestedt, E., Demerath, E., … Loos, R. J. F. (2011). Physical Activity Attenuates the Influence of FTOVariants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children. PLoS Medicine, 8(11), e1001116. http://doi.org/10.1371/journal.pmed.1001116
- Huang, T., Qi, Q., Li, Y., Hu, F. B., Bray, G. A., Sacks, F. M., … Qi, L. (2014). FTOgenotype, dietary protein, and change in appetite: the Preventing Overweight Using Novel Dietary Strategies trial. The American Journal of Clinical Nutrition, 99(5), 1126–1130. http://doi.org/10.3945/ajcn.113.082164
- Yang, X., Yin, L., Li, T., & Chen, Z. (2014). Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPα and PPARγ. International Journal of Clinical and Experimental Medicine, 7(12), 4906–4914.
- Wei Ren, Jianjin Guo, Feng Jiang, et al., 2014 “CCAAT/Enhancer-Binding Protein αIs a Crucial Regulator of Human Fat Mass and Obesity Associated Gene Transcription and Expression,” BioMed Research International, vol. 2014, https://doi.org/10.1155/2014/406909.
- Loos, R. J. F., Lindgren, C. M., Li, S., Wheeler, E., Zhao, J. H., Prokopenko, I., … Mohlke, K. L. (2008). Common variants near MC4Rare associated with fat mass, weight and risk of obesity. Nature Genetics, 40(6), 768–775. http://doi.org/10.1038/ng.140
- Goni, L., García-Granero, M., Milagro, F. I., Cuervo, M., & Martínez, J. A. (2018). Phenotype and genotype predictors of BMI variability among European adults. Nutrition & Diabetes, 8, 27. http://doi.org/10.1038/s41387-018-0041-1
- Tao, Y.-X. (2010). The Melanocortin-4 Receptor: Physiology, Pharmacology, and Pathophysiology. Endocrine Reviews, 31(4), 506–543. http://doi.org/10.1210/er.2009-0037
- Klaauw, A. A., Keogh, J. M., Henning, E., Stephenson, C., Kelway, S., Trowse, V. M., . . . Farooqi, I. S. (2016). Divergent effects of central melanocortin signalling on fat and sucrose preference in humans. Nature Communications, 7, 13055. doi:10.1038/ncomms13055
- Meal Frequencies Modify the Effect of Common Genetic Variants on Body Mass Index in Adolescents of the Northern Finland Birth Cohort 1986
Jääskeläinen A, Schwab U, Kolehmainen M, Kaakinen M, Savolainen MJ, et al. (2013) https://dx.doi.org/10.1371%2Fjournal.pone.0073802 - McDaniel, F. K., Molden, B. M., Mohammad, S., Baldini, G., McPike, L., Narducci, P., … Baldini, G. (2012). Constitutive Cholesterol-dependent Endocytosis of Melanocortin-4 Receptor (MC4R) Is Essential to Maintain Receptor Responsiveness to α-Melanocyte-stimulating Hormone (α-MSH). The Journal of Biological Chemistry, 287(26), 21873–21890. http://doi.org/10.1074/jbc.M112.346890
- Common Genetic Variation near MC4R Has a Sex-Specific Impact on Human Brain Structure and Eating Behavior
Horstmann A, Kovacs P, Kabisch S, Boettcher Y, Schloegl H, et al. (2013) Common Genetic Variation near MC4R Has a Sex-Specific Impact on Human Brain Structure and Eating Behavior.PLOS ONE 8(9): e73802. https://doi.org/10.1371/journal.pone.0073802