Research update: COMT val158Met rs4680
Catechol-O-methyltransferase (COMT) is an enzyme which degrades catechols such as catecholamines (dopamine, adrenaline) and catecholestrogens (formed by cytochrome P450 in the liver). It catalyses the transfer of a methyl group from a donor compound (S adenosyl methionine, SAM) onto the oxygen of one of the hydroxyl groups, in the presence of Mg2+ (1).
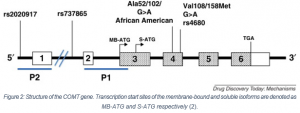
COMT exist in two isoforms, a soluble and a membrane-bound form (S-COMT and MB-COMT, respectively). However, both are encoded on a single COMT gene, found on chromosome 22. The sequence of the two proteins is the same, with MB-COMT having an additional N-terminal section which tethers it to the membrane (2). Both isoforms catalyse the same reaction and have the same active site.
While S-COMT is most abundant in the liver, MB-COMT is predominantly expressed in the brain where it plays a role in dopamine inactivation (3). In some areas of the brain, dopamine transporters readily take up dopamine from the synaptic cleft into the pre-synaptic neuron to terminate the dopamine signal. However, in areas with extremely low levels of these transporters, such as the prefrontal cortex (PFC), COMT plays a much more important role in terminating dopaminergic transmission through dopamine degradation (4).
The rs4680 polymorphism is an extensively studied functional SNP of COMT. A change from G>A results in the conversion of a Valine to Methionine (Val158Met in MB-COMT). The Met allele is associated with a significant reduction in enzyme activity and decreased thermostability (5)(6).
Because of impaired enzyme activity, the Met allele is associated with higher extracellular dopamine levels in the PFC, as dopamine is not degraded as quickly (3). This presumably results in prolonged stimulation of the post-synaptic neuron and/or allows for greater dopamine availability at the synapse.
Memory
This affects influences prefrontal cognitive function, as the prefrontal cortex is an area important for working memory. The association between Val158Met and working memory seemed weak at first, as studies connecting V158Met and working memory have shown mixed results (7–10). However further research focusing on different types of working memory function showed that the SNP has no effect on cognitive processes that only require maintenance of information, but does affect processes demanding active manipulation or updating of information. The Met allele has found to be advantageous here, as it is associated with enhanced cognitive performance (11–15). Thus, Met allele carriers may hold an advantage in memory and attention tasks (16).
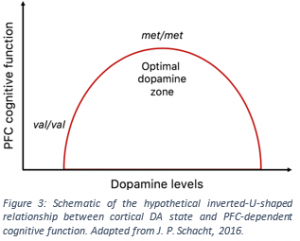
It is believed that there is an optimal intermediate level of dopamine at which prefrontal functioning is most efficient. Too much or too little dopamine seems to have negative effects on working memory. This can be thought of as an inverted-U shaped relationship between prefrontal dopamine levels and PFC performance. As the Met allele is related to higher PFC dopamine levels, Met homozygotes are thought to be closer to the optimum dopamine level on the inverted U curve than Val carriers (Fig 3).
Stress
In contrast, Met allele carriers tend to be more sensitive to biological stressors. Along with demonstrating a greater biological response to stressors (greater hypothalamic-pituitary-adrenal function, greater stress hormone response (17)), Met hetero- and homozygotes have been shown to subjectively experience greater stress in response to a task, compared to Val-homozygotes (18).
Val/val individuals have been shown to respond better to situations of acute and daily stress (19,20).
While Met homozygotes perform better on working memory tasks under no-stress conditions, working memory performance and cognitive function under acute stress is better for val homozygotes (21). It is believed that stress leads to increased release of dopamine and noradrenaline. This can be thought of as a shift to the right along the x-axis in figure 3. Under such conditions, individuals with Val alleles would benefit, having improved dopaminergic transmission and performance. Those with Met alleles would be at a detriment, as they would reach higher than optimal PFC dopamine levels.
There seems to be a trade-off between working memory performance and resiliency to stress. The function of COMT may make Met-carriers more sensitive to stress while improving cognitive performance.
Methylation
The rs4680 SNP is a G>A transition also abolishes a CpG site, where the cytosines are methylation sites. Each Val allele has one CpG methylation site and the Met allele has none. Val/val subjects with greater stress scores have reduced methylation at this site, and better working memory performance. This is most likely because methylation reduces COMT expression. Therefore, silencing of the COMT gene via methylation partially compensates for its negative effect on working memory. In Val/Met subjects, there was no relationship found between methylation, stress, prefrontal cognition, and COMT expression. (22)
Suggested dietary and lifestyle intervention
Further consideration
The manifestation of a COMT allele as an observable phenotype is sometimes influenced by other genes. Deficits in multiple mechanisms produce more pronounced and reliable differences in stress response patterns than functional changes of a single component. These include 5-HTT and MAOA (25,26).
REFERENCES
1. Nissinen E, Männistö PT. Biochemistry and Pharmacology of Catechol-O-Methyltransferase Inhibitors. International review of neurobiology. [Online] 2010. p. 73–118. Available from: doi:10.1016/B978-0-12-381326-8.00005-3 [Accessed: 23rd August 2018]
2. Yager JD. Catechol-O-methyltransferase: characteristics, polymorphisms and role in breast cancer. Drug Discovery Today: Disease Mechanisms. [Online] Elsevier; 2012;9(1–2): e41–e46. Available from: doi:10.1016/J.DDMEC.2012.10.002 [Accessed: 23rd August 2018]
3. Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. American journal of human genetics. [Online] Elsevier; 2004;75(5): 807–821. Available from: doi:10.1086/425589 [Accessed: 23rd August 2018]
4. Käenmäki M, Tammimäki A, Myöhänen T, Pakarinen K, Amberg C, Karayiorgou M, et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. Journal of Neurochemistry. [Online] Wiley/Blackwell (10.1111); 2010;114(6): 1745–1755. Available from: doi:10.1111/j.1471-4159.2010.06889.x [Accessed: 23rd August 2018]
5. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. [Online] 1996;6(3): 243–250. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8807664 [Accessed: 23rd August 2018]
6. Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer research. [Online] 2001;61(18): 6716–6722. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11559542 [Accessed: 23rd August 2018]
7. Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martínez-Larrea A, et al. New Evidence of Association Between COMT Gene and Prefrontal Neurocognitive Function in Healthy Individuals From Sibling Pairs Discordant for Psychosis. American Journal of Psychiatry. [Online] American Psychiatric Publishing; 2004;161(6): 1110–1112. Available from: doi:10.1176/appi.ajp.161.6.1110 [Accessed: 23rd August 2018]
8. Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, et al. Catechol-O-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin Card Sorting Test. Archives of general psychiatry. [Online] 2002;59(7): 662–663. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12090821 [Accessed: 24th August 2018]
9. Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A Functional Polymorphism in the COMT Gene and Performance on a Test of Prefrontal Cognition. American Journal of Psychiatry. [Online] American Psychiatric Publishing; 2002;159(4): 652–654. Available from: doi:10.1176/appi.ajp.159.4.652 [Accessed: 24th August 2018]
10. Wardle MC, de Wit H, Penton-Voak I, Lewis G, Munafò MR. Lack of association between COMT and working memory in a population-based cohort of healthy young adults. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [Online] Nature Publishing Group; 2013;38(7): 1253–1263. Available from: doi:10.1038/npp.2013.24 [Accessed: 24th August 2018]
11. Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive Subprocesses in Working Memory. Archives of General Psychiatry. [Online] 2003;60(9): 889. Available from: doi:10.1001/archpsyc.60.9.889 [Accessed: 24th August 2018]
12. Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, et al. Catechol-O-Methyltransferase (COMT) Genotypes and Working Memory: Associations with Differing Cognitive Operations. Biological Psychiatry. [Online] 2005;58(11): 901–907. Available from: doi:10.1016/j.biopsych.2005.05.010 [Accessed: 24th August 2018]
13. Aguilera M, Barrantes-Vidal N, Arias B, Moya J, Villa H, Ibáñez MI, et al. Putative role of the COMT gene polymorphism (Val158Met) on verbal working memory functioning in a healthy population. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. [Online] 2008;147B(6): 898–902. Available from: doi:10.1002/ajmg.b.30705 [Accessed: 24th August 2018]
14. Costa D de S, de Paula JJ, Alvim-Soares AM, Pereira PA, Malloy-Diniz LF, Rodrigues LOC, et al. COMT Val(158)Met Polymorphism Is Associated with Verbal Working Memory in Neurofibromatosis Type 1. Frontiers in human neuroscience. [Online] Frontiers Media SA; 2016;10: 334. Available from: doi:10.3389/fnhum.2016.00334 [Accessed: 24th August 2018]
15. Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val 108/158 Met genotype on frontal lobe function and risk for schizophrenia. [Online] 2001 [Accessed: 30th August 2018]. Available from: www.pnas.orgcgidoi10.1073pnas.111134598 [Accessed: 30th August 2018]
16. Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS spectrums. [Online] 2006;11(10): 745–748. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17008817 [Accessed: 29th August 2018]
17. Oswald LM, McCaul M, Choi L, Yang X, Wand GS. Catechol-O-methyltransferase polymorphism alters hypothalamic-pituitary-adrenal axis responses to naloxone: a preliminary report. Biological Psychiatry. [Online] Elsevier; 2004;55(1): 102–105. Available from: doi:10.1016/j.biopsych.2003.07.003 [Accessed: 30th August 2018]
18. Hernaus D, Collip D, Lataster J, Ceccarini J, Kenis G, Booij L, et al. COMT Val158Met Genotype Selectively Alters Prefrontal [18F]Fallypride Displacement and Subjective Feelings of Stress in Response to a Psychosocial Stress Challenge. Le Foll B (ed.) PLoS ONE. [Online] Public Library of Science; 2013;8(6): e65662. Available from: doi:10.1371/journal.pone.0065662 [Accessed: 30th August 2018]
19. Collip D, van Winkel R, Peerbooms O, Lataster T, Thewissen V, Lardinois M, et al. COMT Val158Met-Stress Interaction in Psychosis: Role of Background Psychosis Risk. CNS Neuroscience & Therapeutics. [Online] Wiley/Blackwell (10.1111); 2011;17(6): 612–619. Available from: doi:10.1111/j.1755-5949.2010.00213.x [Accessed: 30th August 2018]
20. Walder DJ, Trotman HD, Cubells JF, Brasfield J, Tang Y-L, Walker EF. Catechol-O-methyltransferase modulation of cortisol secretion in psychiatrically at-risk and healthy adolescents. Psychiatric Genetics. [Online] 2010;20(4): 166–170. Available from: doi:10.1097/YPG.0b013e32833a1ff3 [Accessed: 30th August 2018]
21. Buckert M, Kudielka BM, Reuter M, Fiebacj CJ. The COMT Val158Met polymorphism modulates working memory performance under acute stress. Psychoneuroendocrinology. [Online] Pergamon; 2012;37(11): 1810–1821. Available from: doi:10.1016/J.PSYNEUEN.2012.03.014 [Accessed: 30th August 2018]
22. Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A, et al. Stress-related methylation of the catechol-O-methyltransferase Val 158 allele predicts human prefrontal cognition and activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. [Online] Society for Neuroscience; 2011;31(18): 6692–6698. Available from: doi:10.1523/JNEUROSCI.6631-10.2011 [Accessed: 30th August 2018]
23. James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism 1 , 2. The American Journal of Clinical Nutrition. 2004;80(1): 1611–1617.
24. Zhou J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs of the Future. [Online] 2004;29(12): 1235–1244. Available from: doi:10.1358/dof.2004.029.12.855246
25. Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry. [Online] Nature Publishing Group; 2007;12(5): 483–490. Available from: doi:10.1038/sj.mp.4001975 [Accessed: 29th August 2018]
26. Zannas AS, McQuoid DR, Steffens DC, Chrousos GP, Taylor WD. Stressful life events, perceived stress, and 12-month course of geriatric depression: Direct effects and moderation by the 5-HTTLPR and COMT Val158Met polymorphisms. Stress. [Online] Taylor & Francis; 2012;15(4): 425–434. Available from: doi:10.3109/10253890.2011.634263 [Accessed: 30th August 2018]